

Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic column. The sample is transported through the column by the flow of inert, gaseous mobile phase. The column itself contains a liquid stationary phase which is adsorbed onto the surface of an inert solid.
Have a look at this schematic diagram of a gas chromatograph:
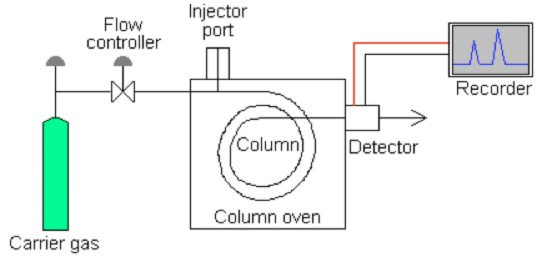
Gas chromatograph columns (GC columns) are tubes through which solutes being measured pass at a rate primarily determined by their physical properties and the temperature and composition of the column. The speed at which solutes move through the column can be weighed against a number of variables to determine its specific properties. As each solute elutes from the column, it enters a heated detector. An electronic signal is generated upon interaction of the solute with the detector. This signal is recorded and plotted.
GC columns are either packed or capillary style. 
Packed GC columns are typically of glass or stainless steel coil construction. They are generally one to five meters in total length with an inner diameter of five millimeters. These devices are filled with a stationary phase, or packing coated with the stationary phase.
Capillary GC columns, also known as open tubular columns, are composed of two major parts: tubing and stationary phase. A thin film (0.1-10.0 micro meters) of high molecular weight, thermally stable polymer is coated onto the inner wall of small diameter tubing, usually 0.05-0.53 mm I.D.
Among capillary columns, there are three sub types to choose from.
Liquid chromatography is a technique used to separate a sample into its individual parts. This separation occurs based on the interactions of the sample with the mobile and stationary phases. Because there are many stationary/mobile phase combinations that can be employed when separating a mixture, there are several different types of chromatography that are classified based on the physical states of those phases. Liquid-solid column chromatography, the most popular chromatography technique and the one discussed here, features a liquid mobile phase which slowly filters down through the solid stationary phase, bringing the separated components with it.
The stationary phase in LC column is most typically a fine adsorbent solid; a solid that is able hold onto gas or liquid particles on its outer surface. The column typically used in column chromatography looks similar to a Pasteur pipette (Pasteur pipettes are used as columns in small scale column chromatography). The narrow exit of the column is first plugged with glass wool or a porous plate in order to support the column packing material and keep it from escaping the tube. Then the adsorbent solid (usually silica) is tightly packed into the glass tube to make the separating column. The packing of the stationary phase into the glass column must be done carefully to create a uniform distribution of material. A uniform distribution of adsorbent is important to minimize the presence of air bubbles and/or channels within the column. To finish preparing the column, the solvent to be used as the mobile phase is passed through the dry column. Then the column is said to be "wetted" and the column must remain wet throughout the entire experiment. Once the column is correctly prepared, the sample to be separated is placed at the top of the wet column. A photo of a packed separating column can be found in the links.
Thank you for taking the time to get to know us better. Kindly submit your requirments. Our Sales Team will contact you at earliest.